Star Death
When we look up at the sky on a dark night, we are seeing essentially the same stars seen by our ancestors at the dawn of human history. Yet, it would be a mistake to conclude that the stars are eternal. Through observations, we have learned that stars have a kind of life cycle of their own, one that is vastly longer than a human lifetime, or even the existence of whole civilizations.
All stars generate their energy through the process of nuclear fusion. The plentiful supply of hydrogen gas in the star, when crushed at high pressures and heated to extreme temperatures, forges heavier elements, like helium or carbon, releasing a tremendous amount of energy in the process. This energy heats up the star, causing it to glow brightly.
White dwarf matter is so dense that a teaspoon of it would weigh as much as a few elephants!
The vast hydrogen supply in a star is not limitless, however, and eventually all stars will come to an end.
The most massive stars burn through their fuel rapidly, at least by astronomical standards, playing out their evolution over mere millions of years. Smaller stars like our Sun will shine for around 10 billion years, and smaller stars will live for longer still. Most stars reach a gentle end, but the largest have a more explosive finale.
It is the ongoing cycle of star birth and star death that has made the Universe into the place we know today, and we owe our very existence to the generations of stars that have come before us. Observing the often spectacular results of a star’s demise using infrared light unlocks some of these mysteries of the process of cosmic recycling that helped make the Earth and everything on it, including us.
Cosmic Recycling
Remarkably, since Edwin Hubble first discovered that everything we can see was expanding in the aftermath of an initial Big Bang, astronomers have put together a pretty amazingly detailed picture of the Universe back to its earliest moments.
After the Big Bang, the Universe was hot and dense, and filled only with hydrogen, helium, and a tiny trace of lithium. That was it. But today we find a rich periodic table of elements across our planet and the cosmos. If we only started with hydrogen and helium, where did the other elements come from?
Nuclear fusion is the process by which lighter elements can be smashed together, fusing into larger, more massive elements. We know this is the process that lights up the Sun and other stars. Amazingly, it seems that all of the heavier elements in the Universe trace back to the nuclear fires of long-gone stars.
When stars die, they recirculate some of their material back into the Universe, and that carries with it a mix of heavier elements that were not present when they formed originally. Gas and dust clouds enriched by these newly-formed elements then provide the building blocks for new stars and solar systems.
The astronomer Carl Sagan famously stated, "We are made of star-stuff," connecting the matter in our bodies to the previous generations of stars in which it was formed.
It turns out that stars have two different ways of participating in the cosmic recycling program. One is relatively quiet, the other strikingly violent. It all depends on the size of the star.
Small Stars: Planetary Nebulae
When smaller stars like our Sun reach the end of their hydrogen-burning lives, one of their final acts is to cast off their outer layers back into interstellar space, forming what we call a “planetary nebula.” The term dates back hundreds of years to the astronomer William Herschel, the discoverer of infrared light. He discovered round, fuzzy objects that, to him, looked a bit like the planet Uranus. While today we know they are not planets, the term is still used.
The story of a planetary nebula begins as the star nears its end. After billion of years of fusing hydrogen into helium, its helium-rich core becomes hot and dense enough to become a fuel source itself. Helium burns to forge an even heavier mix of carbon, nitrogen and oxygen, and this renewed burst of energy puffs the star out into a vastly larger red giant.
If the star is small enough, these heavier elements will never reach the burning point themselves and the fusion process will stop. The star stops producing energy and dies, but in those final stages it sheds its outer layers. This material blows out into interstellar space, carrying with it traces of the heavier elements it once formed, primarily carbon.
The surviving core of the star is known as a “white dwarf.” It is incredibly dense, with most of the mass of the star crammed into an object about the size of the Earth. While it no longer generates energy, it is still very hot and shines brightly in the ultraviolet.
The light from the white dwarf core heats up the surrounding nebula, causing the various atoms and molecules to glow in the infrared. Infrared observations not only help us understand the gases contained in these nebulae, but reveal the carbon dust that can not be seen in visible light.
The infrared view of the Helix nebula has revealed some of the turbulent aftermath following this star’s death. Blue-green colors indicate the outer gassy layers blowing into space. The brighter red circle in the very center is the glow of a dusty disk circling the white dwarf (the disk itself is too small to be seen). Before the star died, its comets (and possibly planets) would have orbited the star in an orderly fashion. But when the star blew off its outer layers, the icy bodies and outer planets would have been tossed about and into each other, resulting in an ongoing cosmic dust storm.
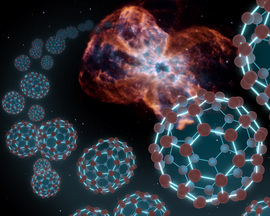
Cosmic Soccer Balls
Planetary nebulae are also home to a very unique kind of carbon molecule, known as "Buckyballs." Buckyballs, short for "Buckminsterfullerene," are spherical molecules made up of 60 carbon atoms. They have a similar structure to classic soccer balls.
Buckyballs were first identified chemically in the lab in 1985 while scientists were simulating the conditions found in the outer envelopes of red giant stars. A quarter of a century later, astronomers using NASA’s Spitzer Space Telescope found them in planetary nebulae, which are the cast-off shells of red giants. In some sense, they were exactly where we expected them to be!
Infrared light was necessary for identifying the unique spectral signature of the Buckyballs. Every atom and molecule can be identified by a precise set of spectral lines that it will emit; these can be seen as a bright glow at specific wavelengths of light. In the case of Buckminsterfullerene, those particular spectral lines appear in the infrared part of the spectrum.
Planetary nebulae provide one important mechanism for stars to recirculate heavier elements back into the galaxy. The next generation of stars that are created gain these materials to help form planets, and in our case on Earth, the many life forms found here. This can help provide carbon, oxygen, and nitrogen, but not the many other heavier elements.
Where, then, do things like silicon, lead, and iron come from?
Big Stars: Supernova Remnants
The most massive stars end their lives rather more spectacularly than gently puffing out a planetary nebula. Instead they can explode so violently that they can briefly outshine the combined light of the rest of the galaxy, and in the process, provide a full spread of heavy elements for the next wave of stars and planets.
Stars that are a bit more massive than our Sun have cores that are compressed, under gravity, to far greater pressures and higher temperatures. In these stars, the carbon becomes a burning fuel source, leaving behind denser oxygen. In fact many heavier elements can be forged, up to the point that an iron core is formed.
The increasingly dense cores of these stars eventually reach a tipping point where the core can no longer hold itself up under the immense crush of gravity. In an instant the core collapses under the gravitational pressure, and the upper layers of the star fall into the void. This incredible release of gravitational energy slams everything together and triggers a massive explosion. The shock wave rebounds through the infalling material, blasting much of it back out into space.
So much energy is released in this explosion that elements much heavier than iron can be formed. Indeed, all of the heaviest elements in the world around us, from lead to gold to uranium, can be traced back to supernova explosions predating the formation of our Solar System.
Published: 13 June, 2014